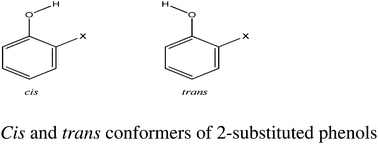
The Abraham solute hydrogen bond acidity parameter A can be derived both from physical methods, A(Gen) and NMR experiments, A(NMR) and results for a large number of hydroxylic solutes show that the two methods agreed very well. However for halophenols the values of A(NMR) were not consistent with the A(Gen) values. The values of A(NMR) suggest that there is no intra-molecular hydrogen bonding in any of the 2-halophenols. In contrast the values of A(Gen) indicate that there is no intra-molecular hydrogen bonding in 2-fluorophenol, but weak intra-molecular hydrogen bonding in 2-chloro, 2-bromo, and 2-iodo-phenol. In view of this uncertainty in the presence or absence of intra-molecular H-bonds in the 2-halophenols, a detailed investigation of the methods used in the literature is presented together with a novel NMR method to determine the ratio of cis and trans forms in these compounds. The experimental data is complemented by a detailed theoretical analysis of the structures and bonding in these molecules to assess the presence or absence of an intra-molecular H-bond. We conclude that there is weak hydrogen bonding in 2-chloro, 2-bromo and 2-iodophenol but very little in 2-fluorophenol.