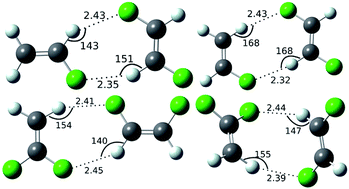
Fluorine bound to a carbon atom (C–F group) behaves differently than its heavier analogues. Weak interactions involving C–F groups in crystal structures have been found to be of immense interest in recent literature. Herein, a series of dimers of ethylene and fluoroethylene have been studied by an ab initio method to calculate the stabilization energy offered by weak C–H⋯F interactions with benchmark accuracy by using a complete basis set (CBS) extrapolation technique. The model complexes have been studied in a systematic fashion to explore the structural and electronic parameters. The total interaction energies of all the complexes have been decomposed to obtain information about the nature of such interactions. Dispersion energy has been found to be the major component in the stabilization energy and C–H⋯F interactions have been found to be of closed shell type in the atoms in molecules (AIM) framework.