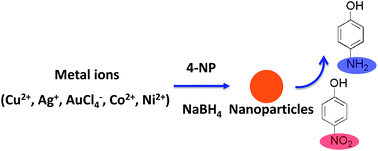
Developing robust and facile catalytic systems for converting nitroaromatic compounds to NH2-containing compounds are of importance to decrease or even eliminate their toxicity or risk in the environment. In view of in situ formed metal nanoparticles, the metal ion (Cu2+, Ag+, AuCl4−, Co2+ and Ni2+)/NaBH4 systems were employed to catalyze the reduction reaction of nitroaromatic compounds. By employing the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) as a model reaction, the effects of concentration of NaBH4, 4-NP and metal ions on the rate constants of the catalytic reduction reactions were systematically investigated. Apparent activation energies of these metal ion/NaBH4 catalytic systems were further measured and compared. In situ formed metal NPs could be characterized by X-ray diffraction (XRD) and transmission electron microscopy (TEM). Furthermore, these metal ion/NaBH4 systems were successfully employed to catalyze the reduction reaction of a series of other nitroaromatic compounds. These metal ion/NaBH4 catalytic systems investigated in this protocol are simple and do not require the preparation of metal nanoparticles in advance, compared with previous related reports.