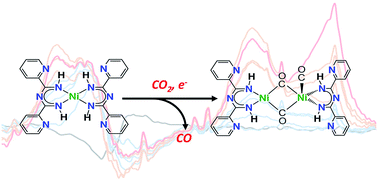
We report the synthesis of Ni(TAPPy)2 (TAPPy = 1,3,5-triazapentadienyl-2,4-bis(2-pyridyl)) and its reactivity with CO2 under reducing conditions. Electrochemical reduction of Ni(TAPPy)2 under inert gas reveals that the complex accommodates up to two additional electrons, with DFT calculations indicating that electron density is delocalized almost exclusively onto the TAPPy ligand framework. The singly reduced product [K(crypt)][Ni(TAPPy)2] (crypt = 2.2.2-cryptand) has been synthesized, and its EPR data is consistent with having ligand-based radical anion character. Controlled potential electrolysis experiments reveal that reduced Ni(TAPPy)2 converts CO2 to form CO; however, spectroscopic and computational data indicate that deactivation readily occurs to form Ni(L)(CO)n compounds, CO32−, and carboxylated (RCOO−) ligand decomposition products. This study highlights that redox activity at the ligand can play an important role during the reduction of CO2 using transition metal complexes.