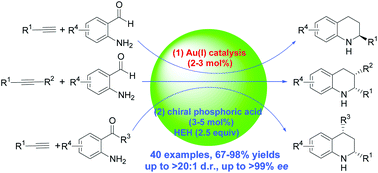
One-pot sequential asymmetric reactions of aminobenzaldehydes or aminophenones with alkynes catalysed by a gold(I)/Brønsted acid cooperative system are reported. This process provides a highly efficient method for the synthesis of optically active tetrahydroquinolines, with one or two chiral centres at different positions as well as highly divergent functional groups, in good to excellent yields and with high regio-, diastereo- and enantioselectivities. A preliminary study on the effect of stereochemistry on biological activity suggests a potential application of these optically active tetrahydroquinolines in drug discovery processes.