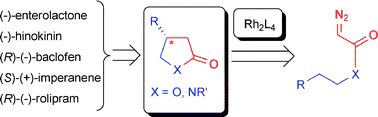
The synthetic potential of highly directional formal insertion of a carbene between carbon and hydrogen of a carbon–hydrogen bond has recently been developed for intramolecular reactions that lead to compounds of biological and medicinal interest. Stereoselective and regiocontrolled intramolecular processes from diazoacetate reactants, catalyzed by dirhodium(II) compounds with chiral carboxamidate ligands, provide efficient and selective access to compounds as diverse as enterolactone, baclofen, imperanene, xylolactone, and rolipram. A comparison of the C–H insertion methodology with alternative approaches is presented.