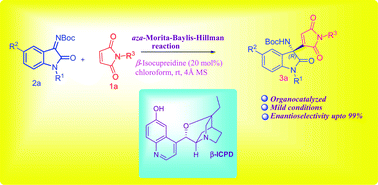
A highly enantioselective Morita–Baylis–Hillman reaction of maleimides with isatin derived ketimines has been developed to obtain enantiomerically enriched 3-substituted-3-aminooxindoles using β-isocupreidine as an organocatalyst. Maleimide acting as a nucleophile provides products with up to 99% ee.