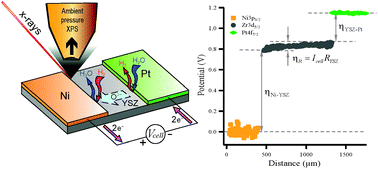
We use photo-electrons as a non-contact probe to measure local electrical potentials in a solid-oxide electrochemical cell. We characterize the cell in operando at near-ambient pressure using spatially-resolved X-ray photoemission spectroscopy. The overpotentials at the interfaces between the Ni and Pt electrodes and the yttria-stabilized zirconia (YSZ) electrolyte are directly measured. The method is validated using electrochemical impedance spectroscopy. Using the overpotentials, which characterize the cell's inefficiencies, we compare without ambiguity the electro-catalytic efficiencies of Ni and Pt, finding that on Ni H2O splitting proceeds more rapidly than H2 oxidation, while on Pt, H2 oxidation proceeds more rapidly than H2O splitting.