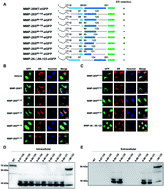
Matrix metalloproteinase 26 (MMP-26), also called endometase and matrilysin-2, belongs to the MMP superfamily. Previous studies have focused on its role in tumor invasion and migration but detailed subcellular localization of MMP-26 remains poorly understood. In this study, sequence deletion mutants of MMP-26 revealed that residues 88–123 function to localize MMP-26 to the endoplasmic reticulum (ER). Moreover, using homologous recombination, we show that exchanging residues 88–123 of secretory MMP-7 with the same region in MMP-26 causes localization of this MMP-7 construct to the ER. Moreover, two (N64, N221) of the three possible N-glycosylation sites in MMP-26 were shown to be N-glycosylated, and N-glycosylation is not required for ER localization. These results demonstrate that the 88–123 region of MMP-26 is a noncanonical ER retention signal and MMP-26 is an N-glycosylated protein, thereby providing novel insights into the properties of MMP-26 within the cell.