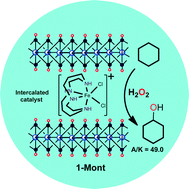
A non-heme iron complex cis-[FeIII(cyclam)Cl2]Cl (cyclam = 1,4,8,11-tetraazacyclotetradecane) has been intercalated into smectite montmorillonite K-10. The intercalated solid has been characterized using EDXRF, AAS, TGA, PXRD, IR and UV-visible analyses. The heterogeneous iron(III)–cyclam has been found to be capable of hydroxylating C–H bonds as strong as those in cyclohexane (BDE = 99.7 kcal mol−1) using benign H2O2 at room temperature. The reactivity of the heterogeneous catalytic system is found to significantly improve in comparison with that of cis-[FeIII(cyclam)Cl2]Cl/H2O2 under homogeneous conditions. The catalytic reactions are marked by a very high selectivity for alcohols which is comparable with the best known non-heme catalysts with H2O2. The results critically reflect the role of the clay matrix surrounding the cationic metal complex in tuning the catalytic activity and selectivity of alkane hydroxylation.