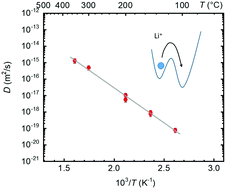
The LiNi0.33Mn0.33Co0.33O2 compound is one of the most interesting cathode materials for Li-ion batteries. Li diffusion in this material directly influences charging/discharging times (and consequently power densities), maximum capacities, stress formation and possible side reactions. In the present study Li tracer self-diffusion is investigated in polycrystalline sintered bulk samples with an average grain size of about 50 nm in the temperature range between 110 and 350 °C. For analysis, stable 6Li tracers are used in combination with Secondary Ion Mass Spectrometry (SIMS). The diffusivities can be described by the Arrhenius law with an activation enthalpy of (0.85 ± 0.03) eV, which is interpreted as the migration energy of a single Li vacancy. Lithium diffuses via structural vacancies whose concentration is fixed by a Li deficiency of about 10%. An extrapolation of the diffusivities to room temperature gives significantly lower values than the diffusivities obtained by electrochemical measurements in literature.