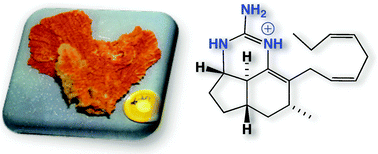
Chemical investigation of a southern Australian marine sponge, Clathria sp., yielded the known mirabilins C, F and G, together with three new analogues, mirabilins H–J. For the first time mirabilins C and F are documented as the underivatized natural products, and a complete absolute stereochemistry is assigned to mirabilin F. Mirabilin I represents the first member of this structure class to incorporate a trans-fused ring junction. Structures for all mirabilins are assigned on the basis of detailed spectroscopic analysis. A plausible polyketide origin is proposed, linking all mirabilins and related sponge alkaloids. Mirabilin cytotoxicity against several human cancer cell lines is discussed.