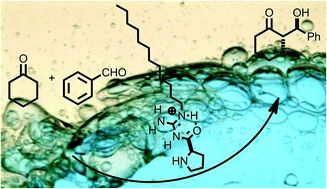
A novel amphiphilic guanidine organocatalyst, efficient for asymmetric aldol reactions of ketones in water at neutral pH, is disclosed. The reaction presented a clear substrate dependence depicting a free energy linear correlation with ee. Intramolecular hydrogen bonding in the acylguanidine moiety was identified as the key structural motif.