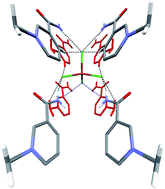
Two series of nicotinamide based ionic liquid crystalline compounds, 3-carbamoyl-1-alkylpyridin-1-ium salts ([Nia–Cn]2X, X = CuCl42− and ZnCl42−; n = 12, 14 and 16), have been synthesized and characterized by single crystal and powder X-ray diffraction, DSC and POM. Both series form interdigitated bilayers in the solid state and liquid crystalline phase. The ionic layers of the two salts are stabilized mainly by four N–H⋯Cl and six C–H⋯Cl hydrogen bonds per anion and has sacrificed the formation of strong N–H⋯O![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonding in the solid state. However, the N–H⋯Cl and N–H⋯O
C hydrogen bonding in the solid state. However, the N–H⋯Cl and N–H⋯O![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonds dominate in the mesophase at high temperature to cause a significant increase of the thickness of the ionic layer.
C hydrogen bonds dominate in the mesophase at high temperature to cause a significant increase of the thickness of the ionic layer.
![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonding in the solid state. However, the N–H⋯Cl and N–H⋯O
C hydrogen bonding in the solid state. However, the N–H⋯Cl and N–H⋯O![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonds dominate in the mesophase at high temperature to cause a significant increase of the thickness of the ionic layer.
C hydrogen bonds dominate in the mesophase at high temperature to cause a significant increase of the thickness of the ionic layer.