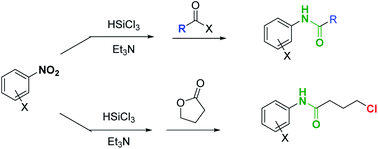
A two-step one pot, experimentally simple protocol, based on readily available and inexpensive reagents allowed the conversion of nitro-arenes directly to N-aryl amides. A metal-free reduction of the nitro group, mediated by trichlorosilane, followed by the addition of an anhydride afforded the corresponding N-aryl carboxyamide, that was isolated after a simple aqueous work up in good-excellent yields. When the methodology was applied to the reaction with γ-butyrolactone, the desired N-aryl butanamide derivative was obtained, featuring a chlorine atom at the γ-position, a functionalized handle that can be used for further synthetic manipulation of the reaction product. Such an intermediate has already been employed as a key advanced precursor of pharmaceutically active compounds.