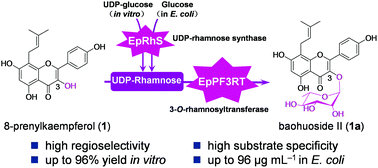
Epimedium is used in traditional Chinese medicine and contains flavonol glycosides that exhibit multiple biological activities. These bioactive flavonol glycosides usually have a rhamnose moiety at the 3-OH position of prenylflavonols, such as icariin (9), baohuoside I (1a) and baohuoside II (2a). However, to date, no rhamnosyltransferase has been reported to catalyze the 3-O-rhamnosylation of prenylflavonols. In this article, a flavonol rhamnosyltransferase, EpPF3RT, was identified from E. pseudowushanense B. L. Guo. The recombinant enzyme regiospecifically transfers a rhamnose moiety to 8-prenylkaempferol (1) and anhydroicaritin (2) at the 3-OH position to form baohuoside II (1a) and baohuoside I (2a) in vitro. In addition, a UDP-rhamnose synthase gene, EpRhS, from E. pseudowushanense was functionally characterized and used to produce the UDP-rhamnose sugar donor. Furthermore, an engineered Escherichia coli strain containing EpPF3RT and EpRhS was established to produce baohuoside II (1a) from whole cells. These studies indicate the significant potential of an enzymatic approach for the rhamnosylation of bioactive flavonoids in Epimedium plants and will provide a promising alternative for producing bioactive rhamnosylated flavonoids combined with other genes/enzymes by synthetic biology.