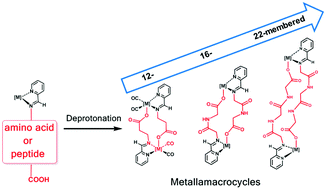
Metallamacrocycles of 12, 16, and 22 members are obtained by deprotonation of the carboxylic group of the side chain of iminopyridine complexes derived from the amino acid β-alanine, and the peptides Gly–Gly and Gly–Gly–Gly. Instead of the expected intramolecular attack to give tridentate (N,N,O) ligands, the deprotonated carboxylate attacks in an inter-molecular manner to give dimers in which the ligand acts as a bridge bonded in a κ2(N,N′) chelating fashion to one metal and as κ(O) to the other metal. The formation of the dimers is supported by NMR spectroscopy, mass spectrometry and X-ray crystallography.