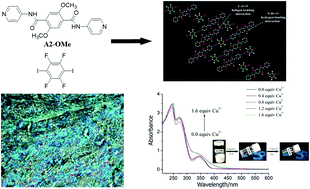
Terephthalic acid-based aromatic amides A1 and A2 and a terephthalaldehyde Schiff-base SB are synthesized, allowing stable gelation with numerous types of organic solvents. Moreover, A2 and SB are liquid crystals with a good mesomorphic temperature domain. The three compounds are halogen bonding (XB) acceptors and form stable XB with 1,4-diiodotetrafluorobenzene (DIB), as proven through Fourier transform infrared (FTIR) spectroscopy, 1H nuclear magnetic resonance titration, and single crystal X-ray analysis of the cocrystal formed by the control compound A2-OMe with DIB. The formation of XB significantly promotes the gelling abilities of A1 and A2, improves the thermal stability of SB, and affects the mesomorphic properties of A2 and SB to a certain degree. Gels formed using A2 or A2 and DIB exhibit selective sensing of Cu2+ over Zn2+, Mg2+, and Hg2+. Scanning electron microscopy, X-ray diffraction, FTIR spectroscopy, polarized optical microscopy, and differential scanning calorimetry are used to study the structures of the gels and mesophases.