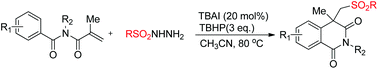An efficient metal-free oxidative arylsulfonylation of α,β-unsaturated imides with sulfonylhydrazides leading to isoquinoline-1,3(2H,4H)-dione derivatives has been developed. The procedure involves the generation of sulfonyl radicals via cleavage of the S–N bond of sulfonylhydrazides with sulfonylation and C–H functionalization. The protocol uses the economical and environmentally friendly TBAI–TBHP catalytic system, and the corresponding isoquinoline-1,3(2H,4H)-diones with various functional groups were obtained in moderate to good yields.