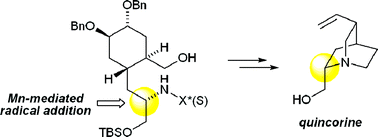
Stereocontrolled Mn-mediated radical addition of alkyl iodides to chiral N-acylhydrazones enables strategic C–C bond disconnection of chiral amines. This strategy was examined in the context of a total synthesis of quinine, generating new findings of functional group compatibility leading to a revised strategy. Completion of a formal synthesis of quinine is presented, validating the application of Mn-mediated radical addition as a useful new C–C bond construction method for alkaloid synthesis. The Mn-mediated addition generates the chiral amine substructure of quinine with complete stereocontrol. Subsequent elaboration includes two successive ring closures to forge the azabicyclo[2.2.2]octane ring system of quincorine, linked to quinine through two known reactions.