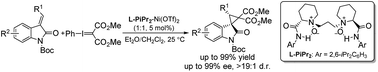
A chiral Lewis acid-promoted enantioselective cyclopropanation using phenyliodonium ylide as the carbene precursor was developed. A variety of spirocyclopropane-oxindoles with contiguous tertiary and all carbon quaternary centers were obtained in excellent outcomes (up to 99% yield, >19 : 1 d.r., up to 99% ee). EPR spectroscopy study supported a stepwise biradical mechanism.