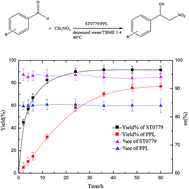
This work demonstrates that the thermophiles can be a rich source to mine catalytic promiscuity, whereby an acyl-peptide releasing enzyme from Sulfolobus tokodaii (ST0779) is identified to be a promising biocatalyst to mediate the Henry (nitroaldol) reaction. Compared to Porcine Pancreatic Lipase (PPL), ST0779 displayed superior catalytic efficiency kcat/Km (6–8 fold higher) and enantioselectivity ee% (90–99%). The catalytic versatility of ST0779 was validated as the enzyme displayed activity towards a broad scope of substituted benzaldehydes, and the electron effects of the benzaldehyde substituents were analyzed by Hammett plotting. Accordingly, this work not only presents a novel enzyme capable of catalyzing the Henry reaction with higher yield and enantioselectivity than ever reported, but also demonstrates the huge potential of Thermophilic archaea to be an optimal source for mining novel enzymes for biocatalytic promiscuity, which could provide a variety of potent biosynthesis tools to yield diverse kinds of molecules.