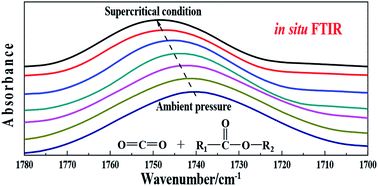
To study the microscopic dispersion state of CO2 in different ester solvents, the solubility, volume expansion coefficients and in situ Fourier transform infrared (FTIR) spectra of the CO2–ester system were measured. The results show that the solubility and expansion coefficient of CO2 in ester solvents decreases as the hydrocarbon chain increases. As the pressure increases, the infrared absorption peaks of CO2 and the functional groups characteristic of ester molecules shift, indicating that CO2 molecules interact with ester molecules and that CO2 would destroy the interactions between the ester molecules. The hydrocarbon chain length of the ester molecules has a significant effect on the infrared absorption peak of the CO2–ester system. As the hydrocarbon chain length increases, the CO2 absorption peak shift and peak shift of the carbonyl groups in the ester gradually decrease.