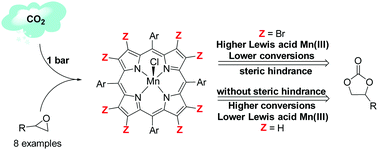
A series of eight Mn(III)–porphyrin (MnP) complexes with electron-withdrawing substituents at the meso and/or β-pyrrole positions of the macrocycle was designed to uncover electronic and structural aspects of MnP catalytic activity in the cycloaddition of CO2 with epoxides. The complexes, when combined with tetrabutylammonium halides, were active catalysts producing the respective cyclic carbonate under mild conditions. The non-β-brominated complex H3[MnT4CPP] served as a structural framework for the design of a series of homologous complexes, leading to the synthesis of the new β-brominated catalysts H3[Mn(BrxT4CPP)] (x = 2, 4, or 6). The β-brominated catalyst series allowed the investigation of the influence of structural effects versus electronic effects on the catalytic system, demonstrating a good correlation between the catalytic activity and the number of bromine substituents at the β-pyrrole positions. The non-planar distortions of the macrocycle and the consequent steric hindrance are determinant for the reaction outcome. The decrease in catalytic activity despite the increase in Lewis acidity of the metal center highlighted the effect of the out-of-plane distortion on the catalytic activity of manganese porphyrins.