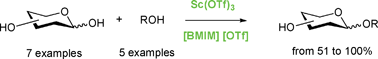Straightforward glycosylation of various alcohols with unprotected and non-activated monosaccharides were performed under scandium triflate catalysis. Rate and yield of glycosylation were highly improved when using 1-butyl-3-methylimidazolium trifluoromethanesulfonate as a green solvent. This ionic liquid was allowed to be recycled at least three times without loss of activity. The possibility of drastically reducing the amounts of catalyst (down to 1 mol%) and aglycone (down to 1 equiv) when performing the reaction in ionic liquid opens new perspectives in O-glycosylation, as a direct coupling between an aglycone and free sugars.