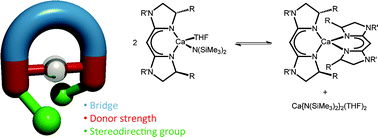
A series of calcium complexes supported by chiral bisimidazoline ligands have been studied in the catalytic intramolecular hydroamination/cyclisation of amino-olefins. The complexes [Ca(R-BIM){N(SiMe3)2}(THF)] (R = 4-C6H4Me, 5a, 4-C6H4F, 5b and tBu, 5c) have given competitive enantioselectivities (up to 12%) when compared to current literature studies involving calcium. Bisimidazolines offer a significant advance over similar bisoxazoline ligands, by allowing a greater structural variance through a modular synthetic pathway.