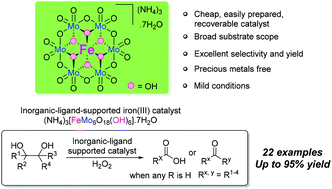
The carbon–carbon bond cleavage of 1,2-diols is an important chemical transformation. Although traditional stoichiometric and catalytic oxidation methods have been widely used for this transformation, an efficient and valuable method should be further explored from the views of reusable catalysts, less waste, and convenient procedures. Herein an inorganic-ligand supported iron catalyst (NH4)3[FeMo6O18(OH)6]·7H2O was described as a heterogeneous molecular catalyst in acetic acid for this transformation in which hydrogen peroxide was used as the terminal oxidant. Under the optimized reaction conditions, carbon–carbon bond cleavage of 1,2-diols could be achieved in almost all cases and carboxylic acids or ketones could be afforded with a high conversion rate and high selectivity. Furthermore, the catalytic system was used efficiently to degrade renewable biomass oleic acid. Mechanistic insights based on the observation of the possible intermediates and control experiments are presented.