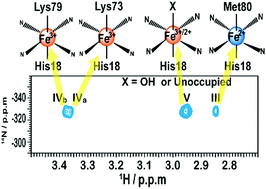
Spectral overlap makes it difficult to use NMR for mapping the conformational profile of heterogeneous conformational ensembles of macromolecules. Here, we apply a 1H–14N HSQC experiment to monitor the alkaline conformational transitions of yeast iso-1 cytochrome c (ycyt c) at natural isotopic abundance. Trimethylated Lys72 of ycyt c is selectively detected by a 1H–14N HSQC experiment, and used as a probe to trace conformational transitions of ycyt c under alkaline conditions. It was found that at least four different conformers of ycyt c coexisted under alkaline conditions. Besides the native structure, Lys73 or Lys79 coordinated conformers and a partially unfolded state with exposed heme were observed. These results indicate that the method is powerful at simplifying spectra of a trimethylated protein, which makes it possible to study complex conformational transitions of naturally extracted or chemically modified trimethylated protein at natural isotopic abundance.