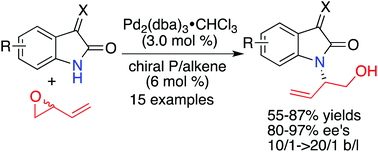
Isatins and their derivatives are important functional moities and building blocks in pharmaceutical and synthetic chemistry. Numerous enantioselective transformations at the C-3 carbonyl group have been well developed. However, the asymmetric substitution reaction with isatins and their derivatives as nucleophiles based on the free N–H groups has been less studied due to the relatively weaker nucleophilicity resulting from the two electron-withdrawing carbonyl groups. In this paper, a palladium-catalyzed asymmetric allylic amination of racemic butadiene monoxide with isatin derivatives using a chiral phosphoramidite olefin hybrid ligand has been successfully developed under mild conditions. A variety of chiral amino alcohols were afforded in 55–87% yields with 10/1->20/1 regioselectivity ratios and 80–97% ees.