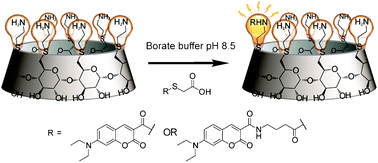
The S → N acylation rates of thioester functionalized coumarins on heptakis-[6-deoxy-6-(2-aminoethylsulfanyl)]-β-cyclodextrin were measured. A high yield of mono-acylation was achieved with products that form self-inclusion compounds. The differential fluorescence response of the functionalized cyclodextrins upon binding biomacromolecules shows the potential of the constructs as probes.