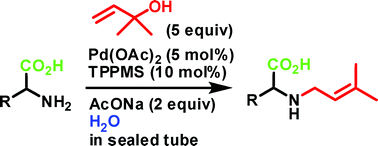
Palladium-catalyzed N-allylation of unprotected amino acids with 1,1-dimethylallyl alcohol were carried out. The reaction in the presence of Pd(OAc)2 (5 mol%), sodium diphenylphosphinobenzene-3-sulfonate (TPPMS, 10 mol%), and AcONa (2 equiv) in water at 120 °C for 16 h in a sealed tube gave only mono-N-allylated amino acids in good yield.