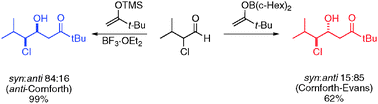
The addition of sterically demanding enolsilanes to α-chloro aldehydes results unexpectedly in preferential formation of the anti-PFA product (1,2-syn), while the addition of the corresponding boron enolate furnishes the expected polar Felkin–Anh product (1,2-anti). A stereoinduction model explaining these observations is proposed.