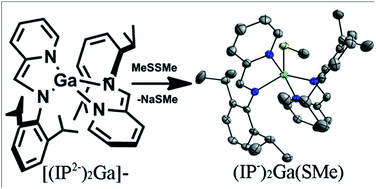
We have prepared a series of gallium(III) complexes of the redox active iminopyridine ligand (IP). Reaction of GaCl3 with iminopyridine ligand (IP) in the presence of either two or four equivalents of sodium metal resulted in the formation of deep green (IP−)2GaCl (1), or deep purple [(DME)3Na][(IP2−)2Ga] (2a), respectively. Complex 1 is paramagnetic with a room temperature magnetic moment of 2.3 μB which falls to 0.5 μB at 5 K. These observations indicate that two ligand radicals comprise a triplet at room temperature which becomes a singlet due to antiferromagnetic coupling at low temperature. Complex 2 is diamagnetic. Cyclic voltammograms recorded on 0.3 M Bu4NPF6 THF solutions of [Na(THF)6][(IP2−)2Ga]− (2b) indicate that oxidation of 2b occurs in two two-electron steps at −1.31 V and −0.54 V vs. SCE. The observation of two-electron redox events indicates that electronic coupling through the gallium(III) center is minimal and that the two IP ligand on 2b are oxidized concurrently. Oxidation of 2 with one equivalent of MeS–SMe afforded the two-electron oxidized product (IP−)2Ga(SMe) (3). This complex has an electronic structure analogous to 1. Accordingly, both 1 and 3 are deep green in color and magnetic susceptibility measurements performed on 3 confirm the triplet character of the complex at room temperature. Electron paramagnetic resonance experiments on 1 and 3 display a quartet signal at g = 2.0 which confirmed the triplet nature of the compounds, and a half field signal consistent with the integer spin state.