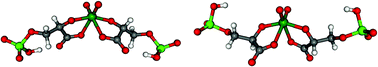
Multinuclear (1H, 13C, 17O, 31P, 95Mo, 183W) magnetic resonance spectroscopy (1D and 2D) has been used to study the complexation of molybdate(VI) and tungstate(VI) with 3-phospho-D-glyceric and 2-phospho-D-glyceric acids. 3-Phospho-D-glyceric acid forms four and five complexes, respectively, with molybdate and tungstate. These have MO22+ centres, and involve the carboxylate and the adjacent OH groups. Two isomeric 1:2 (metal–ligand) complexes are detected, in addition to one mononuclear species having MO3 centres and involving the ligand in a tridentate chelation and a dominant 12:4 species with both tungstate(VI) and molybdate(VI). The dominant 12:4 species can be seen as two 1:2 complexes bound together in a ring through two diphosphometalate moieties, derived from heptamolybdate or heptatungstate, respectively, by inclusion of two phosphate groups from the ligands. Tungstate is also able to form an additional 2:1 tridentate species. 2-Phospho-D-glyceric acid does not interact with tungstate but is able to form one phosphomolybdate species with molybdate, which can be regarded as a heptamolybdate derivative. Density functional theory (DFT) calculations were performed for 1:2 complexes, including calculations on the relative energies of the 1:2 complexes detected in related systems, to validate previously proposed structures. The results are compared with those obtained from multinuclear NMR spectroscopy.