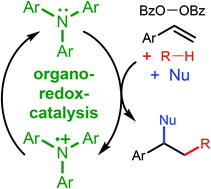
Transition metals are the dominant catalysts for redox-reactions between peroxides and organic substrates. Here, we show that triarylamines can act as organic redox-catalysts, enabling oxidative difunctionalization reactions of alkenes and oxidative Ritter-reactions. Styrene derivatives can be functionalized with alkyl radicals, generated from plain and halogenated hydrocarbons, and with nucleophiles, including nitriles, acetic acid, alcohols and fluoride. An oxidative Ritter reaction can be conducted between allylic C–H bonds as well as fluorene and acetonitrile. Benzoyl peroxide is the oxidant in both reactions. Mechanistic studies suggest that the triarylamines are catalysts and not initiators, mediating the reaction by electron transfer to the peroxide, forming benzoyloxyl radicals, and from C-radical intermediates, forming carbocations.