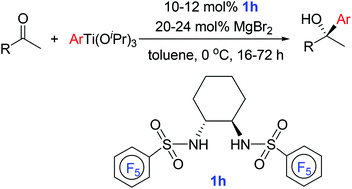
MgBr2-promoted asymmetric addition of ArTi(OiPr)3 to ketones catalyzed by a titanium catalyst of N,N′-sulfonylated (1R,2R)-cyclohexane-1,2-diamines is reported, and results showed that the chiral N,N′-sulfonylated cyclohexane-1,2-diamines with electron-withdrawing groups could effectively catalyze asymmetric addition of ArTi(OiPr)3 to ketones to afford desired tertiary alcohols in good yields with good to excellent enantioselectivities of up to 95% ee.