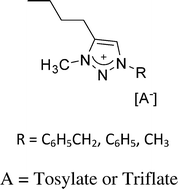
Ionic liquids composed of four different 1,2,3-triazolium cations with tosylate or triflate counter anions have been synthesized and characterized. Physicochemical properties of these ionic liquids including ion cluster behavior, thermal properties, electrochemical stability and ionic conductivity were determined and compared to corresponding imidazolium based ionic liquids. The impact of structure variations, in terms of substituents on the ring of the 1,2,3-triazolium cation and identity of the anion (i.e. tosylate versus triflate) is discussed. Stability of the 1,2,3-triazolium salts towards hydroxide ion at 80 °C was studied. Key features of 1,2,3-triazolium salts are their high electrochemical stability and ionic conductivity, comparable to imidazolium ionic liquids, but better chemical stability under alkaline conditions.