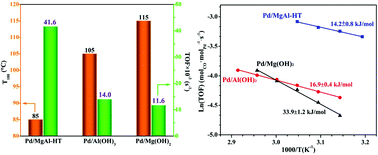
Hydrotalcite-like compounds (HTlcs) are promising supports or catalyst precursors for heterogeneous catalysts. Herein, MgAl-HTlcs-supported Pd catalyst was fabricated, and two Pd catalysts supported on Mg(OH)2 and Al(OH)3 were prepared for comparison. The presence of hydroxyl groups (OH−) in the support is important for obtaining uniform Pd nanoparticles with small sizes. We found that Pdn+ species are more active than Pd0 in low temperature CO oxidation due to their lower barrier in CO activation. The Pd/MgAl-HT catalyst shows the most stable Pdn+ at a temperature lower than 90 °C, leading to the highest catalytic activity towards CO oxidation. Pdn+ in the Pd/Al(OH)3 catalyst is more stable than that in Pd/Mg(OH)2 at low temperature, which is ascribed to its smaller temperature hysteresis (Thysteresis) between the oxidation and re-reduction cycles. The effect of hydroxyl groups on stabilizing Pd species is related to the stability of Pd catalyst in CO oxidation reaction.