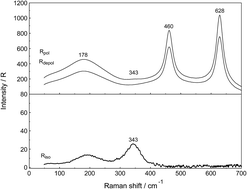
Raman spectroscopic measurements have been made of aqueous solutions of La(ClO4)3, La2(SO4)3, and Na2SO4 in water and heavy water, in the terahertz frequency region (40–1400 cm−1) and down to low concentrations (0.000263 mol L−1). Temperature dependent measurements of a 0.0098 mol L−1 La2(SO4)3 solution have been carried out from 23–98 °C. In solutions of La(ClO4)3 with water and heavy water, the [La(OH2)9]3+ and [La(OD2)9]3+ have been characterized and a weak, strongly polarized band observed at 343 cm−1 and 326 cm−1 respectively assigned to the ν1 LaO9 mode, the breathing mode of the clusters. In La2(SO4)3(aq), in addition to the ν1-SO42− mode at 980 cm−1, a pronounced band component at 991 cm−1 has been assigned to an inner-sphere complex (ISC) and a similar ν1-SO42− band contour has been observed in La2(SO4)3 solutions in D2O. Sulfate may act as a monodentate ligand. Conformation of this assignment is provided by the component at 312 cm−1 of the [La(OH2)8OSO3]+ species in addition to the band at 343 cm−1 for the fully hydrated cluster, [La(OH2)9]3+. After subtraction of the component of the ISC at 991 cm−1, the ν1-SO42− band in La2(SO4)3(aq) showed systematic differences from that in Na2SO4(aq). This is consistent with a ν1-SO42− band at 983.3 cm−1 that can be assigned to the existence of an outer-sphere complex (OSCs). The observed change of the degree of sulfato-complex formation with dilution reflects the stepwise sulfato-complex formation. A K3-value has been determined at 0.9 of the equilibrium between OSC and ISC. Temperature dependent measurements on a dilute La2(SO4)3 solution has shown that the concentration of the La3+ sulfato-complex rises with increasing temperature while at the same time the concentration of the “free” sulfate diminished. The sulfato-complex formation is an endothermic process absorbing heat with increasing temperature. The following thermodynamic parameters for the rate determining equilibrium, [La(OH2)SO4]+ ↔ [LaOSO3]+ has been determined: ΔH0 = 18.6 kJ mol−1 and ΔS0 = 62.1 J mol−1 K−1.