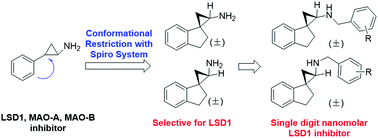
Herein we describe the design, synthesis, and biological evaluation of a novel series of tranylcypromine-based LSD1 inhibitors via conformational restriction using spiro ring systems. A simple, direct spirocyclic analog of tranylcypromine (compounds 8a and 8b) was shown to be a 28- to 129-fold more potent inhibitor of LSD1 enzyme compared to tranylcypromine. Further incorporation of various substituted benzyl groups to the amino group resulted in a suite of 2′,3′-dihydrospiro[cyclopropane-1,1′-inden]-2-amines that are potent LSD1 inhibitors with excellent selectivity profiles (e.g.14a, 15b, 16a, 19a and 20b) against closely related enzymes such as MAO-A, MAO-B, and LSD2.