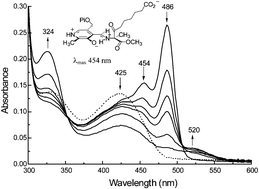
The reactive β-ketoacid pyridoxal-5′-phosphate aldimine formed in the condensation step of the 8-amino-7-oxononanoate synthase reaction was ‘trapped’ in the enzyme-bound form by carrying out the reaction with L-alanine methyl ester and pimeloyl-CoA affording the more stable methyl ester of the putative intermediate, the characterisation of which provides the first definitive evidence for a β-ketoacid intermediate in an α-oxamine synthase mechanism.