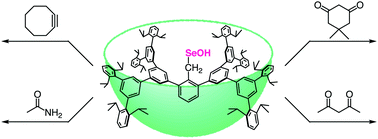
A stable primary-alkyl-substituted selenenic acid was developed as a synthetic model of selenocysteine–derived selenenic acid (Sec–SeOH) by taking advantage of a huge cavity-shaped substituent. The primary-alkyl model compound was successfully applied to model studies on the trapping reaction of protein Sec–SeOH generated in the active site of selenoenzymes with several reagents.