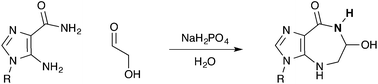
We report an efficient, atom economical general acid–base catalyzed one-step multi-gram synthesis of azepinomycin from commercially available compounds in water. We propose that the described pH-dependent Amadori rearrangement, which couples an amino-imidazole and simple sugar, is of importance as a potential step toward predisposed purine nucleotide synthesis at the origins of life.