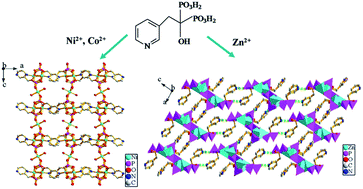
Three new transition metal(II) phosphonates with 3D framework and supramolecular structures, namely, [M1.5(L)(H2O)]·NH2(CH3)2·H2O (M = Ni (1), Co (2)) and [Zn2(H2L)(HL)]·NH2(CH3)2·3H2O (3) (H4L = C5H4NCH2C(OH)(PO3H2)2), have been synthesized under mixed-solvothermal conditions and structurally characterized. Compounds 1 and 2 are isostructural and adopt a 3D framework structure. The {M(1)O5N} octahedra and {CPO3} tetrahedra are interconnected into a 1D chain via corner-sharing, which is further linked to adjacent chains through pyridyl rings to form a 2D layer structure. Neighboring layers are bridged through {M(2)O6}, leading to a 3D framework structure with a 1D channel system along the b-axis. For compound 3, the {Zn(1)O4} and {Zn(2)O4} polyhedra are interconnected by phosphonate groups into a 1D double chain, and the double chain is further connected to adjacent chains through hydrogen bonds to form a 2D supramolecular network. Then these neighboring layers are further connected through hydrogen bonding interactions to give rise to a 3D supramolecular structure. Surface photovoltage spectroscopy (SPS) and field-induced surface photovoltage spectroscopy (FISPS) of compounds 1 and 2 indicate that they possess a positive SPV response in the range of 300–800 nm and show p-type semiconductor characteristics. Compound 3 has been realized for the sensitive sensing of N,N-dimethylformamide (DMF) by a luminescent method.