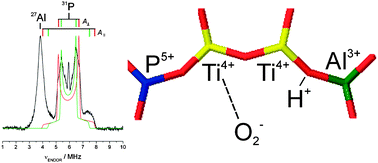
Continuous Wave (CW), pulse Electron Paramagnetic Resonance (EPR) and pulse Electron Nuclear Double Resonance (ENDOR) spectroscopies, in conjunction with UV-Vis and Infrared (IR) spectroscopies, are used to investigate the chemical reactivity of tetrahedrally coordinated Ti3+ ions isomorphously substituted in the framework of AlPO-5 towards NH3 and O2. The coordination of ammonia to Ti3+ centres is followed in detail by complementary vibrational and electron magnetic resonance techniques. In particular HYSCORE spectra allow identifying the coordination of two ammonia molecules to Ti3+ centres resolving the full hyperfine and quadrupole 14N coupling tensors. The reactivity of the reduced TiAlPO sample towards molecular oxygen is detailed by means of CW-EPR and pulse ENDOR spectroscopy. 17O2 is employed, allowing to establish the formation of a “side-on” η2 O2−–Ti4+ electrostatic complex. Pulse ENDOR spectra provide detailed information on the local environment of the formed superoxide radical anion which acts as a paramagnetic probe, providing evidence for Ti–O–Ti oligomeric species.