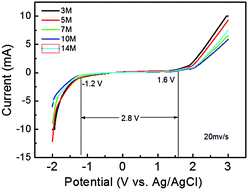
The electrolyte is one of the most essential components for determining the safety, energy density, and cycling performance of energy storage devices, especially supercapacitors. Conventional high-concentration electrolytes have been widely used in high-performance energy storage devices due to their non-flammability and broad working voltage range. Nevertheless, almost all things have two sides; the low electrical conductivity and high viscosity of conventional high-concentration electrolytes commonly restrict the high rate performance of supercapacitors. Herein, propylene carbonate (PC) is introduced into a conventional high-concentration electrolyte system to form a mixed electrolyte of “LiTFSI–PC/H2O”. Compared to the conventional high-concentration electrolyte, this electrolyte has a significantly lower viscosity and higher conductivity without sacrificing the working potential window. The supercapacitor assembled with the 7 M LiTFSI–PC/H2O electrolyte remained at 15 F g−1 after cycling for 10 000 cycles at a current density of 1 A g−1, and the coulombic efficiency was still close to 100%. At the same time, this novel LiTFSI–PC/H2O hybrid electrolyte also reduces costs and improves safety.