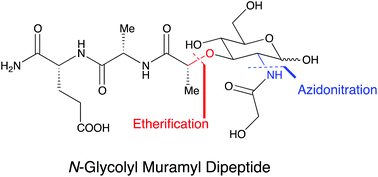
A novel synthetic route leading to N-glycolyl muramyl dipeptide (MDP), a bacterial glycopeptide of particular interest in studies of nucleotide-binding oligomerization domain-containing protein 2 (NOD2), is described. The synthetic strategy hinges on the alkylation of benzylidene-protected glucal with 2-bromopropionic acid and thus circumvents a challenging and non-reproducible SN2 step at the C-3 position of glucosamine derivatives. The subsequent sequence includes an azidonitration and an unusual azide reduction/acylation step via an aza ylide/oxaphospholidine intermediate. This approach generates a protected N-glycolyl MDP that can be either subjected to a one-step global deprotection or differentially deprotected to obtain further derivatives.