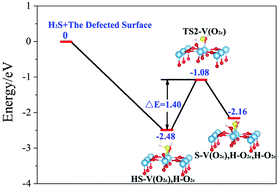
Spin-polarized DFT+U computations have been performed to investigate the role of oxygen vacancies in dissociating H2S on the rutile TiO2(110) surface. A bridged O2c atom is demonstrated to be the most energetically favorable oxygen vacancy site, which makes V(O2c) an electron donator center and induces an isolated defect level with narrowed band gaps. A H2S molecule is adsorbed dissociatively over V(O2c), but molecularly on the perfect surface. For H2S dissociation, the HS/H intermediate state reveals the best thermal stability on both defected and perfect surfaces. Moreover, potential energy surface analysis shows that V(O2c) reduces markedly the energy barriers for the paths along H2S dissociation. This indicates oxygen vacancies to be efficient trap centers for H2S dissociation, as evidenced by a significant interfacial charge transfer promoted by vacancies. This work could provide insights into the role of oxygen vacancies in facilitating the decomposition of H2S on rutile TiO2(110) surface.