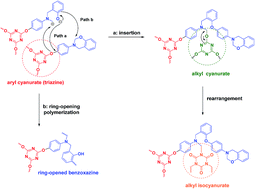
To study the co-reaction of benzoxazine and triazine, a triazine-containing benzoxazine (P-tta) was prepared through nucleophilic substitution of 4-(2H-benzo[e][1,3]oxazin-3(4H)-yl)phenol (P-ap) with 2,4,6-trichloro-1,3,5-triazine. DSC thermograms show that the exothermic temperature of P-tta is lower than that of other benzoxazines with a similar structure except for the triazine structure, so we speculate that the forward polymerization is related to the existence of the triazine structure. Through monitoring the curing process using IR, we propose that the curing reactions of P-tta include a concerted co-reaction between the triazine and benzoxazine, and a self-polymerization of benzoxazine. A thermoset with a high Tg (279 °C, DMA data), a low thermal expansion coefficient (32 ppm per °C), and high thermal stability (Td5% 417 °C) can be obtained through the curing of P-tta.