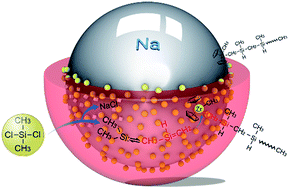
Competition between two different polymerization pathways to polymethylsilanes (PMS) and polycarbosilanes (PCS) was studied. This occurs in a Wurtz reduction-coupling reaction system during dechlorination of several dichloromethylsilanes via sodium in toluene. These two reactions do not carry on pari-passu, i.e., PMS is exclusively formed. However, PCS can be formed dominantly when some zirconocenes are introduced. This competition was introduced as a result of a tautomeric transformation from methylsilylene (MeRSi:) into 1-silene (CH2![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) SiRH). These are formed simultaneously as two intermediates but are influenced by the substitution of methyl, ethyl, phenyl or vinyl groups on silicon in the dichloromethylsilanes. Polymerization of methylsilylenes into PMS can be inhibited by deactivation of the sodium surface via chemical adsorption of zirconocene dichloride. Hence, the catalytic insertion polymerization of 1-silene intermediates into PCS occurs at the active sites of zirconocenes—this leads to the formation of PCS conversion ratios of 82% to 93% from dichloromethylsilanes with methyl, ethyl and phenyl as substituents. However, catalytic polymerization does not happen from dichloromethylvinylsilane. The mechanisms of insertion polymerization as well as the thermal dynamic barrier from polymethylvinylsilane (PMVS) to polyvinylcarbosilane (PVCS) are discussed in detail.
SiRH). These are formed simultaneously as two intermediates but are influenced by the substitution of methyl, ethyl, phenyl or vinyl groups on silicon in the dichloromethylsilanes. Polymerization of methylsilylenes into PMS can be inhibited by deactivation of the sodium surface via chemical adsorption of zirconocene dichloride. Hence, the catalytic insertion polymerization of 1-silene intermediates into PCS occurs at the active sites of zirconocenes—this leads to the formation of PCS conversion ratios of 82% to 93% from dichloromethylsilanes with methyl, ethyl and phenyl as substituents. However, catalytic polymerization does not happen from dichloromethylvinylsilane. The mechanisms of insertion polymerization as well as the thermal dynamic barrier from polymethylvinylsilane (PMVS) to polyvinylcarbosilane (PVCS) are discussed in detail.